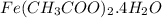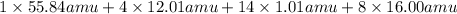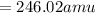Chemistry, 12.03.2020 01:36 Joshuafranklindude
Calculate the molecular (formula) mass of the compound: iron(II) acetate tetrahydrate. A. 186.9 amu B. 245.9 amu C. 191.5 amu D. 242.7 amu E. 197.9 amu

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, officialrogerfp3gf2s
How to solve 4 nh3(g) + 5 o2(g) > 4 no(g) + 6 h2o(g) in chemistry
Answers: 1

Chemistry, 22.06.2019 04:00, clairebear66
What three natural resources are found in the great lakes region
Answers: 2

Chemistry, 22.06.2019 05:30, nuclearfire278
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease. correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1

Chemistry, 22.06.2019 09:10, cheesedoodle
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1
You know the right answer?
Calculate the molecular (formula) mass of the compound: iron(II) acetate tetrahydrate. A. 186.9 amu...
Questions in other subjects:

Mathematics, 27.02.2021 19:50

English, 27.02.2021 19:50


Chemistry, 27.02.2021 19:50