The law of conservation of mass states that in a chemical reaction the mass of the reactants
eq...

Chemistry, 12.03.2020 00:31 GxthGrl6612
The law of conservation of mass states that in a chemical reaction the mass of the reactants
equals the mass of the products. Based on this information, what mass of hydrogen (H2)
was produced in this reaction?
A. 2 g
B. 4 g
C. 72 g
D. 144 g
please leave an explanation
thank you!
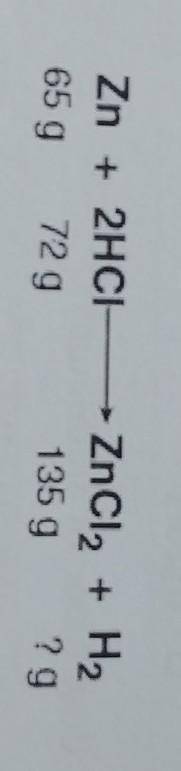

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, yolo123321
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2


Chemistry, 22.06.2019 12:10, kaitlynbernatz2778
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 17:00, emma3216
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1
You know the right answer?
Questions in other subjects:


Mathematics, 16.04.2021 14:00

English, 16.04.2021 14:00

Mathematics, 16.04.2021 14:00


English, 16.04.2021 14:00

Biology, 16.04.2021 14:00

History, 16.04.2021 14:00




