
Chemistry, 11.03.2020 23:39 samantha9014
An aqueous potassium iodate ( KIO3 ) solution is made by dissolving 562 562 grams of KIO 3 KIO3 in sufficient water so that the final volume of the solution is 4.30 L. Calculate the molarity of the KIO 3 KIO3 solution.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:10, bri9263
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3



Chemistry, 22.06.2019 22:30, COOLIOMARIS
What three things does a balanced equation show you?
Answers: 1
You know the right answer?
An aqueous potassium iodate ( KIO3 ) solution is made by dissolving 562 562 grams of KIO 3 KIO3 in s...
Questions in other subjects:

Mathematics, 07.10.2019 13:00

History, 07.10.2019 13:00



Social Studies, 07.10.2019 13:00



English, 07.10.2019 13:00


History, 07.10.2019 13:00

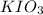 solution is 0.612 mol/L
solution is 0.612 mol/L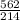 moles of
moles of 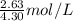 = 0.612 mol/L
= 0.612 mol/L

