
Chemistry, 11.03.2020 22:59 ronaldhernandez598
The compound trimethylamine, (CH3)3N, is a weak base when dissolved in water. Write the Kb expression for the weak base equilibrium that occurs in an aqueous solution of trimethylamine:

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, ulilliareinhart2
The is a particle with one unit of positive charge a. proton b. positron c. electron d. nucleus awnser quick it is a important science test!
Answers: 2

Chemistry, 22.06.2019 02:50, giiffnlojd
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3

Chemistry, 22.06.2019 05:40, yah2muchh
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2
You know the right answer?
The compound trimethylamine, (CH3)3N, is a weak base when dissolved in water. Write the Kb expressio...
Questions in other subjects:


Mathematics, 02.12.2020 05:10



Mathematics, 02.12.2020 05:10

History, 02.12.2020 05:10



Mathematics, 02.12.2020 05:10

Mathematics, 02.12.2020 05:10

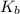 expression for the weak base equilibrium is:
expression for the weak base equilibrium is:![K_b=\frac{[(CH_3)_3NH^+][OH^-]}{[(CH_3)_3N]}](/tpl/images/0543/5992/9ca05.png)
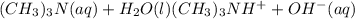
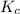 can be given as:
can be given as:![K_c=\frac{[(CH_3)_3NH^+][OH^-]}{[(CH_3)_3N][H_2O]}](/tpl/images/0543/5992/c3722.png)
![K_b=K_c\times [H_2O]=\frac{[(CH_3)_3NH^+][OH^-]}{[(CH_3)_3N]}](/tpl/images/0543/5992/fb8c1.png)
![[H_2O]=1](/tpl/images/0543/5992/b8579.png)
![K_b=K_c\times 1=\frac{[(CH_3)_3NH^+][OH^-]}{[(CH_3)_3N]}](/tpl/images/0543/5992/c4048.png)


