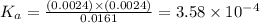Chemistry, 11.03.2020 22:13 msjsnell29
A weak monoprotic acid has molar mass 180 g/mol. When 1.00 g of this acid is dissolved in enough water to obtain a 300 mL solution, the pH of the resulting solution is found to be 2.62. What is the value of Ka for this acid

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, 767sebmont
Temperature and kinetic energy are proportional. a) adirectly b) directly c) indirectly
Answers: 2

Chemistry, 21.06.2019 22:30, andaws21
This large tectonic plate is bounded on three sides by whats know as the ring of fire. what is the name of this tectonic plate? a) pacific plate b) eurasian plate c) north american plate d) indo- australian plate plz it's science but there's no option for science so i picked chemistry
Answers: 2

Chemistry, 22.06.2019 22:30, skinniestoflegends
Consider a culture medium on which only gram-positive organisms such as staphylococcus aureus colonies can grow due to an elevated nacl level. a yellow halo surrounds the growth, indicating the bacterium fermented a sugar in the medium, decreasing the ph as a result and changing the color of a ph indicator chemical. this type of medium would be referred to as a differential and enrichment culture.
Answers: 2

Chemistry, 23.06.2019 07:30, mazielynn84
If you try to move a piano and are unable to move it, did you perform any work in the scientific sense of the word? yes or no?
Answers: 1
You know the right answer?
A weak monoprotic acid has molar mass 180 g/mol. When 1.00 g of this acid is dissolved in enough wat...
Questions in other subjects:

Mathematics, 20.03.2020 01:39

Mathematics, 20.03.2020 01:39






Mathematics, 20.03.2020 01:39

Chemistry, 20.03.2020 01:39

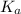 for the given acid is
for the given acid is 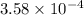
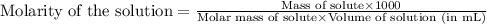
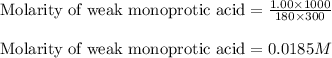
![pH=-\log[H^+]](/tpl/images/0543/4513/cf945.png)
![2.62=-\log[H^+]](/tpl/images/0543/4513/2e60f.png)
![[H^+]=10^{-2.62}=2.40\times 10^{-3}M=0.0024M](/tpl/images/0543/4513/dcf01.png)
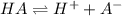
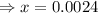
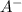 = x = 0.0024 M
= x = 0.0024 M![K_a=\frac{[H^+][A^-]}{[HA]}](/tpl/images/0543/4513/66f51.png)