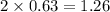At a certain temperature, the equilibrium constant, K c , for this reaction is 53.3. H 2 ( g ) + I 2 ( g ) − ⇀ ↽ − 2 HI ( g ) K c = 53.3 At this temperature, 0.800 mol H 2 and 0.800 mol I 2 were placed in a 1.00 L container to react. What concentration of HI is present at equilibrium?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:40, jeffcarpenter
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1


Chemistry, 22.06.2019 09:40, cheesecake1919
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3
You know the right answer?
At a certain temperature, the equilibrium constant, K c , for this reaction is 53.3. H 2 ( g ) + I 2...
Questions in other subjects:

History, 30.11.2021 22:40

SAT, 30.11.2021 22:40

Business, 30.11.2021 22:40



SAT, 30.11.2021 22:40


History, 30.11.2021 22:40


SAT, 30.11.2021 22:40

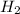 = 0.800 mole
= 0.800 mole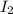 = 0.800 mole
= 0.800 mole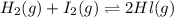
![K_c=\frac{[HI]^2}{[H_2]\times [l_2]}](/tpl/images/0543/3494/7dfaa.png)
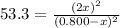
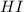 at equilibrium = 2 x =
at equilibrium = 2 x =