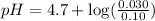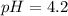Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, peaceouthjkdrb2398
Rutherford's experiment indicated that matter was not as uniform as it appears what part of his experimental results implied this idea
Answers: 1



You know the right answer?
What is the pH of a solution prepared by mixing 30.00 mL of 0.10 MCH3CO2H with 30.00 mL of 0.030 MCH...
Questions in other subjects:

Social Studies, 08.12.2021 19:50

History, 08.12.2021 19:50

Social Studies, 08.12.2021 19:50


Mathematics, 08.12.2021 19:50

Biology, 08.12.2021 19:50

Geography, 08.12.2021 19:50



History, 08.12.2021 19:50

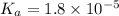
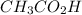 = 0.10 M
= 0.10 M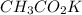 = 0.030 M
= 0.030 M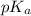 .
.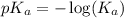
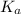 in this expression, we get:
in this expression, we get: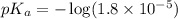
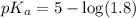
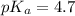
![pH=pK_a+\log \frac{[Salt]}{[Acid]}](/tpl/images/0543/3032/e961a.png)
![pH=pK_a+\log \frac{[CH_3CO_2K]}{[CH_3CO_2H]}](/tpl/images/0543/3032/8b433.png)