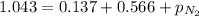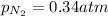Chemistry, 11.03.2020 18:22 makrosebud7821
A mixture of He, NE, and N2 gases has a pressure of 1.043 atm. If the pressures of He and Ne are 0.137 atm and 0.566 atm, respectively, what is the partial pressure of N2 in the mixture?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:00, smelcher3900
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2

Chemistry, 22.06.2019 17:00, brownvester44
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

You know the right answer?
A mixture of He, NE, and N2 gases has a pressure of 1.043 atm. If the pressures of He and Ne are 0.1...
Questions in other subjects:

Mathematics, 13.07.2019 19:00

Mathematics, 13.07.2019 19:00

Mathematics, 13.07.2019 19:00

Mathematics, 13.07.2019 19:00

Mathematics, 13.07.2019 19:00


Social Studies, 13.07.2019 19:00


Mathematics, 13.07.2019 19:00

Mathematics, 13.07.2019 19:00

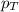 = total pressure of gas = 1.043 atm
= total pressure of gas = 1.043 atm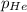 = partial pressure of helium gas = 0.137 atm
= partial pressure of helium gas = 0.137 atm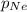 = partial pressure of neon gas = 0.566 atm
= partial pressure of neon gas = 0.566 atm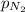 = partial pressure of nitrogen gas = ?
= partial pressure of nitrogen gas = ?