
Chemistry, 11.03.2020 17:49 tylerkitchen44
What volume (in L) of oxygen will be required to produce 77.4 L of water vapor in the reaction below?
2c2H6(g)+7O2(g)--->4CO2(g)+6H2O( g)
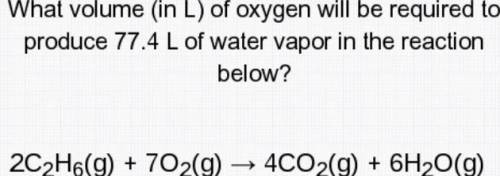

Answers: 3


Other questions on the subject: Chemistry



You know the right answer?
What volume (in L) of oxygen will be required to produce 77.4 L of water vapor in the reaction below...
Questions in other subjects:




History, 06.01.2021 17:10




Mathematics, 06.01.2021 17:10

Mathematics, 06.01.2021 17:10

Mathematics, 06.01.2021 17:10



