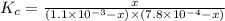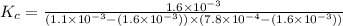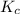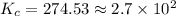Consider the following reaction:
Fe3+(aq)+SCN−(aq) <> FeSCN2+(aq)
A solution is ma...

Chemistry, 11.03.2020 02:42 Chewbacka2020
Consider the following reaction:
Fe3+(aq)+SCN−(aq) <> FeSCN2+(aq)
A solution is made containing an initial [Fe3+] of 1.1 x 10^−3 M and an initial [SCN−] of 7.8 x 10^−4 M . At equilibrium, [FeSCN2+]= 1.6 x 10^−4 M .
Part A) Calculate the value of the equilibrium constant (Kc).
Express your answer using two significant figures.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:10, board1692
3.) for each of the following compounds, draw the major organic product of reaction with hcl or naoh and circle whether the starting materials and products will be more soluble in organic solvent or water benzoic acid + hcl: benzoic acid + naoh: oh benzoic acid water/organic water organic fluorenone hс: fluorenone + naoh: fluorenone water/organic water/organic веnzocaine + hci: benzocaine + n»oh: h2n benzocaine water/organic water organic o=
Answers: 3

Chemistry, 22.06.2019 05:30, livigrace9004
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2


Chemistry, 22.06.2019 19:30, simihehe
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2
You know the right answer?
Questions in other subjects:


Chemistry, 20.11.2020 19:20

Mathematics, 20.11.2020 19:20


Mathematics, 20.11.2020 19:20



Mathematics, 20.11.2020 19:20


 .
.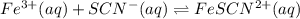
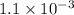
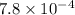 0
0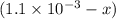
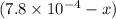 x
x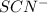 at equilibrium is given ,x=
at equilibrium is given ,x=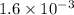
![K_c=\frac{[FeSCN^{2+}]}{[Fe^{3+}][SCN^-]}](/tpl/images/0542/1843/417f5.png)