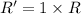In acidic solution, the breakdown of sucrose into glucose and fructose has this rate law: rate = k[H+][sucrose].
The initial rate of sucrose breakdown is measured in a solution that is 0.01 M H+, 1.0 M sucrose, 0.1 M fructose, and 0.1 M glucose.
How does the rate change if:
(a) [Sucrose] is changed to 2.5 M?
(b) [Sucrose], [fructose], and [glucose] are all changed to 0.5 M?
(c) [H+] is changed to 0.0001 M?
(d) [Sucrose] and [H+] are both changed to 0.1 M ?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:50, trinityrae4657
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 23.06.2019 03:30, vaehcollier
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 1

Chemistry, 23.06.2019 12:10, rainbowboy9231
What is the correct name for hg(no3)2? mercury (i) nitrate mercury (ii) nitrate mercury nitroxide mercury dinitride
Answers: 1

Chemistry, 23.06.2019 13:00, jacobanderson43
What type of reaction is this equation c2h5s + o2 > co2 + h2o + so2
Answers: 2
You know the right answer?
In acidic solution, the breakdown of sucrose into glucose and fructose has this rate law: rate = k[H...
Questions in other subjects:

History, 26.09.2021 14:00


Mathematics, 26.09.2021 14:00

Mathematics, 26.09.2021 14:00



History, 26.09.2021 14:00

English, 26.09.2021 14:00

Social Studies, 26.09.2021 14:00

Social Studies, 26.09.2021 14:00

![[H^+]](/tpl/images/0542/2176/07acb.png) is changed to 0.0001 M than rate will be increased by the factor of 0.01.
is changed to 0.0001 M than rate will be increased by the factor of 0.01.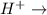 fructose+ glucose
fructose+ glucose![R=k[H^+][sucrose]](/tpl/images/0542/2176/8ca17.png)
![[H^+]=0.01M](/tpl/images/0542/2176/8ae83.png)
![R=k[0.01M][1.0 M]](/tpl/images/0542/2176/7a749.png) ..[1]
..[1]![R'=[0.01 M][2.5 M]](/tpl/images/0542/2176/5a418.png) ..[2]
..[2]![\frac{R'}{R}=\frac{[0.01 M][2.5 M]}{k[0.01M][1.0 M]}](/tpl/images/0542/2176/ac5ef.png)
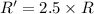
![R'=[0.01 M][0.5 M]](/tpl/images/0542/2176/d8698.png) ..[2]
..[2]![\frac{R'}{R}=\frac{[0.01 M][0.5 M]}{k[0.01M][1.0 M]}](/tpl/images/0542/2176/036aa.png)
![R'=[0.0001 M][1.0 M]](/tpl/images/0542/2176/62405.png) ..[2]
..[2]![\frac{R'}{R}=\frac{[0.0001 M][1.0M]}{k[0.01M][1.0 M]}](/tpl/images/0542/2176/7dc89.png)
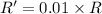
![R'=[0.1M][0.1M]](/tpl/images/0542/2176/67bb4.png) ..[2]
..[2]![\frac{R'}{R}=\frac{[0.1M][0.1M]}{k[0.01M][1.0 M]}](/tpl/images/0542/2176/6860b.png)