
Chemistry, 11.03.2020 02:25 unicornpoop54
A certain chemical reaction releases 480. kJ of heat energy per mole of reactant consumed. Suppose some moles of the reactant are put into a calorimeter (a device for measuring heat flow). It takes 3.85 J of heat energy to raise the temperature of this calorimeter by 1 °C. Now the reaction is run until all the reactant is gone, and the temperature of the calorimeter is found to rise by 14.6 °C. How would you calculate the number of moles of reactant that were consumed? Set the math up. But don't do any of it. Just leave your answer as a math expression. Also, be sure your answer includes all the correct unit symbols. moles consumed-

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 22:10, leo4687
What does a particular point on a line of a phase diagram represent? o a. the maximum temperature a substance can exist at without bonds breaking b. the pressure created by the kinetic energy of molecules at a particular temperature c. the melting point or boiling point of a substance at a specific pressure d. the conditions in which temperature and pressure have equal effects on a substance
Answers: 2

Chemistry, 23.06.2019 03:00, amberskids2
You have a sample of a metal, the sample is exactly 6.02 x 1023atom, if the sample has a mass 55.85 what metal is your sample made of?
Answers: 2
You know the right answer?
A certain chemical reaction releases 480. kJ of heat energy per mole of reactant consumed. Suppose s...
Questions in other subjects:

Mathematics, 11.12.2020 01:10

Chemistry, 11.12.2020 01:10


Advanced Placement (AP), 11.12.2020 01:10

Mathematics, 11.12.2020 01:10

Mathematics, 11.12.2020 01:10



Mathematics, 11.12.2020 01:10

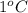 . So here, specific heat is given as 3.85 J. And, the change in temperature is
. So here, specific heat is given as 3.85 J. And, the change in temperature is 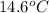 .
.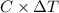
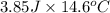
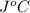
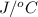 of heat is calculated as follows.
of heat is calculated as follows.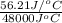
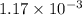 moles
moles

