
At a certain temperature this reaction follows second-order kinetics with a rate constant of Suppose a vessel contains at a concentration of . Calculate the concentration of in the vessel seconds later. You may assume no other reaction is important. Round your answer to significant digits.

Answers: 3


Other questions on the subject: Chemistry




Chemistry, 22.06.2019 21:30, jpimentel2021
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2
You know the right answer?
At a certain temperature this reaction follows second-order kinetics with a rate constant of Suppose...
Questions in other subjects:

Mathematics, 05.02.2021 03:40


Mathematics, 05.02.2021 03:40


Mathematics, 05.02.2021 03:40





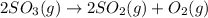
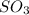 in the vessel after 0.240 seconds is 0.24 M
in the vessel after 0.240 seconds is 0.24 M![k=\frac{1}{t}\left (\frac{1}{[A]}-\frac{1}{[A]_o}\right)](/tpl/images/0541/9921/5ea71.png)
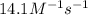
![[A]_o](/tpl/images/0541/9921/9caf5.png) = Initial concentration = 1.44 M
= Initial concentration = 1.44 M![14.1=\frac{1}{0.240}\left (\frac{1}{[A]}-\frac{1}{1.44}\right)](/tpl/images/0541/9921/8dfd8.png)
![[A]=0.245M](/tpl/images/0541/9921/0a4f3.png)


