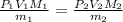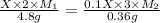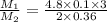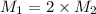Suppose you are given two flasks at the same temperature, one of volume 2 L and the other of volume 3 L. The 2-L flask contains 4.8 g of gas, and the gas pressure is X atm. The 3-L flask contains 0.36 g of gas, and the gas pressure is 0.1X. Do the two gases have the same molar mass? If not, which contains the gas of higher molar mass?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:00, angeljohnson2081
Which object forms when a supergiant runs out of fuel? a red giant a black hole a white dwarf a neutron star
Answers: 1

Chemistry, 22.06.2019 04:00, armahoney8566
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2

You know the right answer?
Suppose you are given two flasks at the same temperature, one of volume 2 L and the other of volume...
Questions in other subjects:

Mathematics, 21.07.2019 08:30

Mathematics, 21.07.2019 08:30

Biology, 21.07.2019 08:30

Geography, 21.07.2019 08:30




English, 21.07.2019 08:30


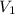 = 2 L,
= 2 L, 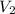 = 3 L,
= 3 L, 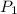 = X,
= X, 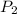 = 0.1 X,
= 0.1 X,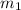 = 4.8 g,
= 4.8 g, 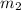 = 0.36 g,
= 0.36 g,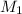 = ?,
= ?, 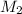 = ?
= ?