
Chemistry, 10.03.2020 22:29 chickenwing32
For the reaction 2 HCl + Na2CO3 → 2 NaCl + H2O + CO2 8.0 moles of CO2 is collected at STP. What is the volume of CO2? 1) 57.6 L 2) 22.4 L 3) 0.0250 L 4) 2.80 L 5) 179 L 6) 0.357 L

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, alexmarche4675
Mrs. smith ordered a root beer float (vanilla ice cream + root beer). mrs. smith noticed that the three states of matter (solid, liquid, and gas) all existed simultaneously in her root beer float. a. identify each phase of matter in the root beer float. b. describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? ) c. identify one phase change you would see in a root beer float and described what causes this change.
Answers: 2

Chemistry, 22.06.2019 01:00, XxrazorxX11
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1

Chemistry, 22.06.2019 12:30, johnsont8377
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1

Chemistry, 22.06.2019 14:00, hannahhoskings6989
What was bohr’s contribution to the planetary model
Answers: 1
You know the right answer?
For the reaction 2 HCl + Na2CO3 → 2 NaCl + H2O + CO2 8.0 moles of CO2 is collected at STP. What is t...
Questions in other subjects:




Mathematics, 25.08.2021 22:30

Health, 25.08.2021 22:30

Mathematics, 25.08.2021 22:30




Mathematics, 25.08.2021 22:30

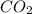 = 8.0 mole
= 8.0 mole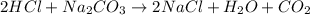
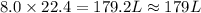 volume of
volume of 

