
Consider the following mechanism for the oxidation of bromide ions by hydrogen peroxide in aqueous acid solution. H+ + H2O2 ? H3O2+ (rapid equilibrium) H3O2+ + Br- ? HOBr + H2O (slow) HOBr+H+ +Br- ?Br2 +H2O(fast) Which rate law is consistent with this mechanism?
a. k[Br-][H+]-1[H2O2]-1
b. k[H+][H2O2][Br-]
c. k[H+][H2O2]
d. k[HOBr][H+][Br-]

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:30, breannaking9734
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2


You know the right answer?
Consider the following mechanism for the oxidation of bromide ions by hydrogen peroxide in aqueous a...
Questions in other subjects:


Mathematics, 19.03.2020 06:00

Mathematics, 19.03.2020 06:00


English, 19.03.2020 06:01





![\text{Rate}=k'[H+][H_2O_2][Br^-]](/tpl/images/0541/5354/e35f3.png)
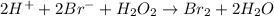


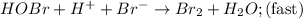
![\text{Rate}=k[H_3O_2^+][Br^-]](/tpl/images/0541/5354/37d2f.png) ......(1)
......(1)![[H_3O_2^+]](/tpl/images/0541/5354/85c88.png) is not appearing as a reactant in the overall reaction. So, we apply steady state approximation in it.
is not appearing as a reactant in the overall reaction. So, we apply steady state approximation in it.![K=\frac{[H_3O_2^+]}{[H^+][H_2O_2]}](/tpl/images/0541/5354/4cd3a.png)
![[H_3O_2^+]=K[H^+][H_2O_2]](/tpl/images/0541/5354/15cd9.png)
![\text{Rate}=k.K[H^+][H_2O_2][Br^-]\\\\\text{Rate}=k'[H+][H_2O_2][Br^-]](/tpl/images/0541/5354/f918c.png)


