
At a certain temperature the rate of this reaction is first order in HI with a rate constant of :7.21s?1
2HI(g)??H2(g) + I2(g)
Suppose a vessel contains HI at a concentration of 0.440M
. Calculate the concentration of HI in the vessel 0.210 seconds later. You may assume no other reaction is important.
Round your answer to 2 significant digits.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, MickeyxX7096
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3

Chemistry, 22.06.2019 09:00, tbiles99
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 17:30, shookiegriffin
I'm learning about the periodic tables and what each subject's configuration is. for example, hydrogen is 1s^1, but i don't understand how you get that. can someone me understand how to figure out how to figure this out? sorry if the question makes no sense, but it would really a lot if you could me understand! you so much if you can!
Answers: 1
You know the right answer?
At a certain temperature the rate of this reaction is first order in HI with a rate constant of :7.2...
Questions in other subjects:




Mathematics, 08.10.2019 22:00


Mathematics, 08.10.2019 22:00

History, 08.10.2019 22:00

Biology, 08.10.2019 22:00

Biology, 08.10.2019 22:00

Mathematics, 08.10.2019 22:00

![[A_o]=0.440 M](/tpl/images/0541/4407/bdc04.png)
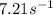
![[A]=[A_o]\times e^{-kt}](/tpl/images/0541/4407/abdec.png)
![[A]=0.440 M\times e^{-7.21 s^{-1}\times 0.210 s}](/tpl/images/0541/4407/4243c.png)


