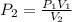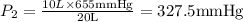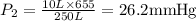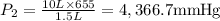Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:30, tiffanyhmptn
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1

Chemistry, 22.06.2019 23:30, sweaversw9602
What are the similarities between compounds and mixtures?
Answers: 3

Chemistry, 23.06.2019 05:30, hongkongbrat6840
Astudent made the lewis dot diagram of a compound as shown. mg is written with two dots shown on its top. an o is written on each side of mg. each o has six dots around it. an arrow is shown from one dot on mg toward the vacant space around the o on the right. another arrow is shown from the other dot on mg toward the vacant space around the o on the left. the title of the art is students lewis dot model. what is the error in the lewis dot diagram? an o atom should transfer all its six electrons to mg because the formula is mgo. both electrons of mg should be transferred to one o atom because the formula is mgo. the electrons should be transferred from each o atom to mg because mg has fewer electrons. the number of dots around mg should be four because it has to transfer two electrons to each o.
Answers: 2
You know the right answer?
A 10.0-L balloon contains helium gas at a pressure of 655 mmHg. What is the new pressure, in mmHg, o...
Questions in other subjects:

Mathematics, 27.06.2020 02:01

Geography, 27.06.2020 02:01







Mathematics, 27.06.2020 02:01

 10 = P2
10 = P2