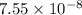Chemistry, 10.03.2020 19:01 topangabraith
Two equilibrium reactions of nitrogen with oxygen, with their corresponding equilibrium constants (Kc) at a certain temperature, are given below. reaction (1): N2(g) + O2(g) equilibrium reaction arrow 2 NO(g); Kc = 2.59e-31 reaction (2): N2(g) + 1/2 O2(g) equilibrium reaction arrow N2O(g); Kc = 3.31e-24 Using this set of data, determine the equilibrium constant for the following reaction, at the same temperature. reaction (3): N2O(g) + 1/2 O2(g) equilibrium reaction arrow 2 NO(g)

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, leslyrivera11
If i make a solution by adding 83grams of sodium hydroxide to 750ml i’d water what is the molarity of sodium hydroxide
Answers: 1

Chemistry, 21.06.2019 22:30, kiki197701
Agas at 155 kpa and standard temperature has an initial volume of 1.00 l. the pressure of the gas rises to 500 kpa as the temperature also rises to 135°c. what is the new volume? 2.16 l 0.463 l 0.207 l 4.82 l
Answers: 3

Chemistry, 21.06.2019 23:30, tylerineedhelp
Ihat will happen if i added baking soda to vinegar
Answers: 2

Chemistry, 22.06.2019 08:30, mosthatedpicky1
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2
You know the right answer?
Two equilibrium reactions of nitrogen with oxygen, with their corresponding equilibrium constants (K...
Questions in other subjects:

Mathematics, 01.04.2020 19:14

Chemistry, 01.04.2020 19:14

Mathematics, 01.04.2020 19:14


English, 01.04.2020 19:14





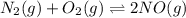
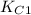 of this reaction is as follows.
of this reaction is as follows.![\frac{[NO]^{2}}{[N_{2}][O_{2}]}](/tpl/images/0541/2546/adeca.png)
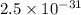
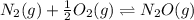
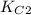 of this reaction is as follows.
of this reaction is as follows.![\frac{[N_{2}O]}{[N_{2}][O_{2}]^{\frac{1}{2}}}](/tpl/images/0541/2546/7b620.png)
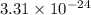
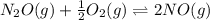
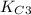 of this reaction is as follows.
of this reaction is as follows.![\frac{[NO]^{2}}{[N_{2}O][O_{2}]^{\frac{1}{2}}}](/tpl/images/0541/2546/c0a44.png)
![\frac{[NO]^{2}}{[N_{2}][O_{2}]} \times \frac{[N_{2}][O_{2}]}{[N_{2}O][O_{2}]^{\frac{1}{2}}}](/tpl/images/0541/2546/93fb5.png)
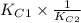
![[tex]2.5 \times 10^{-31} \times \frac{1}{3.31 \times 10^{-24}}](/tpl/images/0541/2546/55cbe.png)