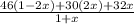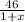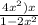Chemistry, 10.03.2020 19:09 kellysmith45
A sample of pure NO2 is heated to 335 ∘C at which temperature it partially dissociates according to the equation 2NO2(g)⇌2NO(g)+O2(g) At equilibrium the density of the gas mixture is 0.525 g/L at 0.750 atm .

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, skaterwolf1317
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 12:30, robert7248
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2
You know the right answer?
A sample of pure NO2 is heated to 335 ∘C at which temperature it partially dissociates according to...
Questions in other subjects:



English, 02.09.2021 20:20


Mathematics, 02.09.2021 20:20


Mathematics, 02.09.2021 20:20

English, 02.09.2021 20:20

Mathematics, 02.09.2021 20:20

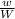 =
= 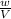 = density
= density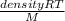 density is 0.525g/L, temperature= 608.15 K, P = 0.750 atm
density is 0.525g/L, temperature= 608.15 K, P = 0.750 atm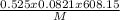
![\frac{ [NO] [O2]}{NO2}](/tpl/images/0541/2934/d8d55.png)
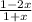 x M NO2 +
x M NO2 + 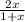 M NO+
M NO+ 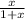 M O2
M O2