

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 20.06.2019 18:04, kelly3215
If a certain battery supplies 1.0×108 electrons per second to the negative terminal and the battery contains 7.00 moles of electrolytic solution (which means the solution contains 7.00 moles of hso4), what fraction of the solution undergoes a chemical reaction each second
Answers: 1

Chemistry, 22.06.2019 11:50, hamidaakter936848
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 17:50, kaylamount
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. start this question by first balance the chemical equation.1.) balance the equation: - alo3(s)+naoh(l)+hf(> na3alf6+h2o(g). 2.) if 17.5 kilograms of al2o3(s), 51.4 kilograms of naoh(l), and 51.4 kilograms of hf(g) react completely, how many kilograms of cryolite will be produced? 3.)which reactants will be in excess, (al2o3, naoh, or hf) 4.)what is the total mass of the excess reactants left over after the reaction is complete in kg?
Answers: 2

Chemistry, 22.06.2019 18:50, christhegreat1
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1
You know the right answer?
With an excess of oxygen gas according to the following chemical equation. 4 P 502 > 2 P2Os What...
Questions in other subjects:

Mathematics, 30.08.2019 07:00


History, 30.08.2019 07:00




Mathematics, 30.08.2019 07:00


Mathematics, 30.08.2019 07:00

English, 30.08.2019 07:00

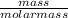
 = 5.55mole
= 5.55mole  = 2.78moles
= 2.78moles 

