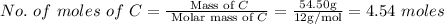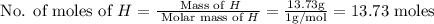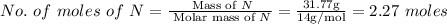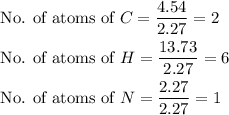The rotten smell of a decaying animal carcass is partially due to a nitrogen-containing compound called putrescine. Elemental analysis of putrescine indicates that it consists of:
C
,
54.50
%
,
H
,
13.73
%
, and
N
,
31.77
%
. Calculate the empirical formula of putrescine.

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 11:30, chelseychew32
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

Chemistry, 22.06.2019 18:50, christhegreat1
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1
You know the right answer?
The rotten smell of a decaying animal carcass is partially due to a nitrogen-containing compound cal...
Questions in other subjects:

Biology, 06.11.2020 04:10

History, 06.11.2020 04:10



History, 06.11.2020 04:10

World Languages, 06.11.2020 04:10


Arts, 06.11.2020 04:10

English, 06.11.2020 04:10

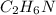 .
.