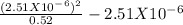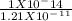Chemistry, 10.03.2020 17:07 heysorryguys
Lactic acid is a weak acid found in milk. Its calcium salt is a source of calcium for growing animals. A saturated solution of this salt, which we can represent as (Ca(Lact)2) has a [Ca2+]=0.26M and a pH=8.40.
Calculate the Ka of lactic acid, assuming the salt is 100% dissociated.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 23.06.2019 00:30, quintink
How can you write e method for the experiment of separating sand from water by filtration process? 1-materials 2-steps 3-conclusion also the same for the separating process of water and salt by filtration or distillation. quick because i need to finish my hw
Answers: 2


Chemistry, 23.06.2019 06:30, nikeahbrown
Consider the heating curve of h2o and line segments a, b, and c. several changes are taking place at a, b, and c. all but one would be an appropriate description as e move through segments a, b and then c.
Answers: 3
You know the right answer?
Lactic acid is a weak acid found in milk. Its calcium salt is a source of calcium for growing animal...
Questions in other subjects:


English, 03.11.2020 02:50


Health, 03.11.2020 02:50

Mathematics, 03.11.2020 02:50


Biology, 03.11.2020 02:50

Arts, 03.11.2020 02:50