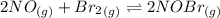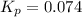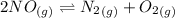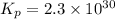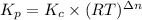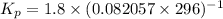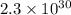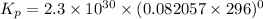Chemistry, 10.03.2020 09:56 mattmaddox86
Calculate the equilibrium constant Kp for this reaction, given the following information (at 296 K ): 2NO(g)+Br2(g)⇌2NOBr(g)Kc=1.8 2NO(g)⇌N2(g)+O2(g)Kc=2.3×1030

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:00, angtrevv
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 17:00, smelcher3900
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2
You know the right answer?
Calculate the equilibrium constant Kp for this reaction, given the following information (at 296 K )...
Questions in other subjects:





Biology, 18.03.2021 02:10

Mathematics, 18.03.2021 02:10



English, 18.03.2021 02:10

Mathematics, 18.03.2021 02:10