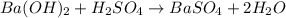For the following reaction, 56.6 grams of barium hydroxide are allowed to react with 30.1 grams of sulfuric acid. barium hydroxide (aq) + sulfuric acid (aq) barium sulfate (s) + water (l) What is the maximum amount of barium sulfate that can be formed? grams What is the FORMULA for the limiting reagent? What amount of the excess reagent remains after the reaction is complete? grams

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, coolkid2041
Calculate the number of moles of ethane in 100 grams
Answers: 3


Chemistry, 22.06.2019 09:00, lrasanaoaksandfurana
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1
You know the right answer?
For the following reaction, 56.6 grams of barium hydroxide are allowed to react with 30.1 grams of s...
Questions in other subjects:







Biology, 28.10.2019 22:31



 .
.