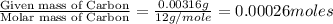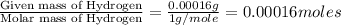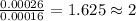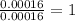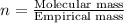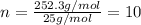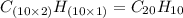Chemistry, 10.03.2020 09:06 auviannadority13
One of the components of natural crude oil and coal deposits is benzo[a]pyrene, a compound with a molecular mass of about 252.3 amu, containing only carbon and hydrogen. A 3.320 mg sample of benzo[a]pyrene burns to give 11.58 mg of CO2. Determine its empirical and molecular formulas. (Omit states-of-matter from your answer.)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, bartfrank447
Joseph has hypothesized that sound travels in waves. if he were following the scientific method, what should he do next? a. ask a question. b. test the hypothesis. c. study the results. d. tell other scientists about his hypothesis.
Answers: 1

Chemistry, 22.06.2019 04:00, eborkins
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3


Chemistry, 23.06.2019 00:00, baseball1525
Which item is most likely part of the safety contract
Answers: 1
You know the right answer?
One of the components of natural crude oil and coal deposits is benzo[a]pyrene, a compound with a mo...
Questions in other subjects:






Social Studies, 29.10.2019 01:31



Mathematics, 29.10.2019 01:31

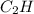 and
and 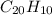
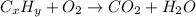
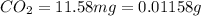 (Conversion factor: 1 g = 1000 mg)
(Conversion factor: 1 g = 1000 mg)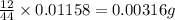 of carbon will be contained.
of carbon will be contained.