

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, deidaraXneji
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3

Chemistry, 22.06.2019 14:10, roserose3098
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2


Chemistry, 22.06.2019 21:30, sullivanjakob
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1
You know the right answer?
If 61.1 mL of lead(II) nitrate solution reacts completely with excess sodium iodide solution to yiel...
Questions in other subjects:

Social Studies, 05.01.2020 02:31


English, 05.01.2020 02:31

Mathematics, 05.01.2020 02:31

Physics, 05.01.2020 02:31

Mathematics, 05.01.2020 02:31

History, 05.01.2020 02:31

Biology, 05.01.2020 02:31


Mathematics, 05.01.2020 02:31

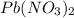 is precipitated as
is precipitated as 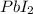 when NaI is added to solution of
when NaI is added to solution of 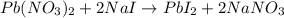
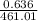 mol of
mol of 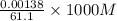 = 0.0226 M
= 0.0226 M

