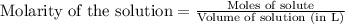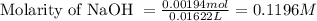A 0.397-g sample of potassium hydrogen phthalate, KHC8H4O4 (molar mass = 204.22 g/mol) is dissolved with 50 mL of deionized water in a 125-mL Erlenmeyer flask. The sample is titrated to the phenolphthalein endpoint with 16.22 mL of a sodium hydroxide solution. What is the molar concentration of the NaOH solution? (Show all calculations)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, brasherfamily14
Which term describes a fracture in the earth at which land stays in the same place? a. joint b. fault c. split d. hinge
Answers: 1



Chemistry, 23.06.2019 02:00, Paytonsmommy09
Butane gas reacts with oxygen gas to give carbon dioxide gas and water vapor (gas). if you mix butane and oxygen in the correct stoichiometric ratio, and if the total pressure of the mixture is 390 mmhg, what is the pressure (in mmhg) of water vapor after the reaction is completed (temperature and volume do not change).
Answers: 2
You know the right answer?
A 0.397-g sample of potassium hydrogen phthalate, KHC8H4O4 (molar mass = 204.22 g/mol) is dissolved...
Questions in other subjects:

Chemistry, 15.10.2020 16:01


Health, 15.10.2020 16:01

Mathematics, 15.10.2020 16:01

English, 15.10.2020 16:01



Mathematics, 15.10.2020 16:01

History, 15.10.2020 16:01

Mathematics, 15.10.2020 16:01

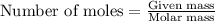
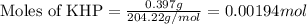
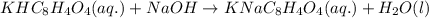
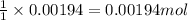 of NaOH.
of NaOH.