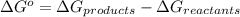For the following reaction, label each of the below species as an acid or a base. Use lower case letters only (e. g. acid)
HCN + HPO4⁻² ⇔ H2PO4⁻ + CN⁻
Will the above reaction take place spontaneously? (Is the reaction product-favored? Does the equilibrium lie to the right?)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, pressure772
Which is a character of nuclear fusion but not nuclear fission
Answers: 3


Chemistry, 22.06.2019 15:00, makaylajones74pdxtrk
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 19:00, innocentman69
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1
You know the right answer?
For the following reaction, label each of the below species as an acid or a base. Use lower case let...
Questions in other subjects:

Mathematics, 12.10.2020 22:01

Spanish, 12.10.2020 22:01


Mathematics, 12.10.2020 22:01


Mathematics, 12.10.2020 22:01

History, 12.10.2020 22:01



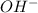 .
. 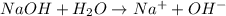
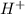 .
.
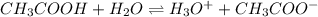
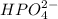 is accepting the hydrogen ions so it acts as a base.
is accepting the hydrogen ions so it acts as a base.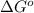 values of the given species are as follows.
values of the given species are as follows.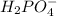 = -1130.4 kJ/mol,
= -1130.4 kJ/mol, 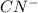 = 172.4 kJ/mol
= 172.4 kJ/mol