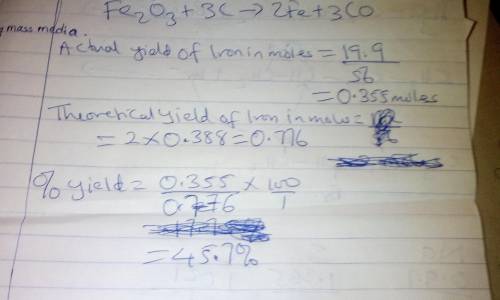Chemistry, 10.03.2020 08:05 quanwalker651370
Combining 0.338 mol Fe 2 O 3 with excess carbon produced 19.9 g Fe . Fe 2 O 3 + 3 C ⟶ 2 Fe + 3 CO What is the actual yield of iron in moles? actual yield: mol What is the theoretical yield of iron in moles? theoretical yield: mol What is the percent yield? percent yield:

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, Makoshark6887
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 06:20, stephliu721
What is a property of a double replacement reaction
Answers: 1
You know the right answer?
Combining 0.338 mol Fe 2 O 3 with excess carbon produced 19.9 g Fe . Fe 2 O 3 + 3 C ⟶ 2 Fe + 3 CO Wh...
Questions in other subjects:

Mathematics, 30.04.2021 01:00



English, 30.04.2021 01:00


Mathematics, 30.04.2021 01:00

Mathematics, 30.04.2021 01:00


Mathematics, 30.04.2021 01:00