Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, angtrevv
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 15:20, mydoggy152
Fossil fuels are organic compounds that are made from
Answers: 1

Chemistry, 22.06.2019 17:30, sheazy3709
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2

Chemistry, 22.06.2019 18:00, liddopiink1
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3
You know the right answer?
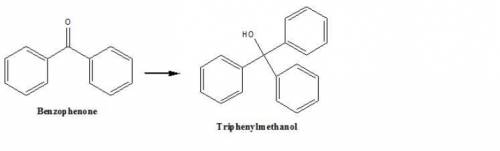
This reaction forms triphenylmethanol from benzophenone. How could you use infrared spectroscopy to...
Questions in other subjects:

Arts, 21.03.2021 17:10


Mathematics, 21.03.2021 17:10


English, 21.03.2021 17:10



English, 21.03.2021 17:10

Mathematics, 21.03.2021 17:10

Mathematics, 21.03.2021 17:10

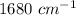 and frequency band of this wavelength is absent in triphenylmethanol.
and frequency band of this wavelength is absent in triphenylmethanol.  and frequency band of this wavelength is absent in benzophenone.
and frequency band of this wavelength is absent in benzophenone.