
Chemistry, 10.03.2020 08:21 lovebunny33921
CO(g)+2H2(g)⇌CH3OH(g)CO(g)+2H2(g)⇌C H3OH(g) This reaction is carried out at a different temperature with initial concentrations of [CO]=0.27M[CO]=0.27M and [H2]=0.49M[H2]=0.49M. At equilibrium, the concentration of CH3OHCH3OH is 0.11 MM. Find the equilibrium constant at this temperature.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 11:00, coco8560
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 23.06.2019 01:30, yarrito20011307
Which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1
You know the right answer?
CO(g)+2H2(g)⇌CH3OH(g)CO(g)+2H2(g)⇌C H3OH(g) This reaction is carried out at a different temperature...
Questions in other subjects:



History, 02.08.2019 01:50


Social Studies, 02.08.2019 01:50

Geography, 02.08.2019 01:50

Mathematics, 02.08.2019 01:50

Mathematics, 02.08.2019 01:50

Chemistry, 02.08.2019 01:50

Mathematics, 02.08.2019 01:50

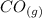 +
+ 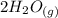 ⇄
⇄ 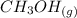
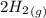 ⇄
⇄ ![K_C = \frac{[CH_3OH]}{[CO][H_2]^2}](/tpl/images/0540/6074/3667b.png)
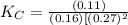
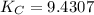
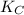 = 9.4
= 9.4

