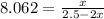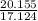Chemistry, 10.03.2020 08:14 depinedainstcom
At 25°C, the equilibrium constant Kc for the reaction 2A(aq) ↔ B(aq) + C(aq) is 65. If 2.50 mol of A is added to enough water to prepare 1.00 L of solution, what will the equilibrium concentration of A be?

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 12:00, sophiaa23
Which of the following is an example of physical change not a chemical change? a) a log gives off heat and light as it burns. b) a tree stores energy from the sun in its fruit. c) a penny lost in the grass slowly changes color. d) a water pipe freezes and cracks on a cold night.
Answers: 2

Chemistry, 22.06.2019 18:30, rosie20052019
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1
You know the right answer?
At 25°C, the equilibrium constant Kc for the reaction 2A(aq) ↔ B(aq) + C(aq) is 65. If 2.50 mol of A...
Questions in other subjects:

Health, 07.04.2021 23:30

English, 07.04.2021 23:30


English, 07.04.2021 23:30

Mathematics, 07.04.2021 23:30

Mathematics, 07.04.2021 23:30


Spanish, 07.04.2021 23:30

Chemistry, 07.04.2021 23:30

Physics, 07.04.2021 23:30

 = 65
= 65
![K_c =\frac {[B][C]}{[A]^2}](/tpl/images/0540/5702/5350b.png)
![65 = \frac{[x][x]}{[2.5-2x]^2}](/tpl/images/0540/5702/bd4f1.png)
![65 = \frac{[x]^2}{[2.5-2x]^2}](/tpl/images/0540/5702/0dd40.png)
![65 = (\frac{[x]}{[2.5-2x]})^2](/tpl/images/0540/5702/0c0f2.png)
![\sqrt 65 = \sqrt {(\frac{[x]}{[2.5-2x]})^2}](/tpl/images/0540/5702/bb4a3.png)