
Chemistry, 10.03.2020 07:28 umimgoingtofail
The heat of fusion of dichloromethane (CH2Cl2) is 6.2 kJ/mol.
Calculate the change in entropy Δs when 345 g of dichloromethane freezes at -95.1 °C.
Be sure your answer contains a unit symbol and the correct number of significant digits.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 23:00, NewKidnewlessons
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3

Chemistry, 23.06.2019 01:00, skatelife8974
What is the chemical name of the compound ti2o3?
Answers: 2

Chemistry, 23.06.2019 01:30, Dmoney5104
The biomedical technique in which a part of the brain is destroyed with electric current is known as a. electroconvulsive therapy b. prefrontal lobotomy c. bilateral cingulotomy d. tardive dyskinesia
Answers: 2
You know the right answer?
The heat of fusion of dichloromethane (CH2Cl2) is 6.2 kJ/mol.
Calculate the change in entropy...
Calculate the change in entropy...
Questions in other subjects:



Mathematics, 04.04.2021 14:00

English, 04.04.2021 14:00



English, 04.04.2021 14:00


Mathematics, 04.04.2021 14:00


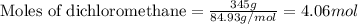

 = Entropy change = ?
= Entropy change = ?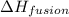 = enthalpy of fusion = 6.2 kJ/mol = 6200 J/mol (Conversion factor: 1 kJ = 1000 J)
= enthalpy of fusion = 6.2 kJ/mol = 6200 J/mol (Conversion factor: 1 kJ = 1000 J)![-95.1^oC=[-95.1+273]K=177.9K](/tpl/images/0540/3534/1b16d.png)



