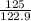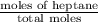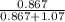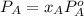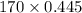Chemistry, 10.03.2020 07:25 starfox5454
At a certain temperature the vapor pressure of pure heptane (C_7 H_16) is measured to be 170. torr. Suppose a solution is prepared by mixing 86.7 g of heptane and 125. g of acetyl bromide (CH_3 COBr). Calculate the partial pressure of heptane vapor above this solution. Round your answer to 3 significant digits. Note for advanced students: you may assume the solution is ideal.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, erickamurillo9929
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2


Chemistry, 22.06.2019 12:30, americanbellabeauty
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

Chemistry, 23.06.2019 08:40, mathisaqeosmw
Calculate the number of grams of sodium in 3.00 g of each sodium-containing food additive.
Answers: 3
You know the right answer?
At a certain temperature the vapor pressure of pure heptane (C_7 H_16) is measured to be 170. torr....
Questions in other subjects:


Chemistry, 15.05.2021 06:00

Mathematics, 15.05.2021 06:00


History, 15.05.2021 06:00

Social Studies, 15.05.2021 06:00



Spanish, 15.05.2021 06:00

Chemistry, 15.05.2021 06:00


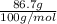
 are calculated as follows.
are calculated as follows.