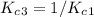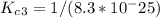Chemistry, 10.03.2020 06:51 Felici6086
Enter your answer in the provided box. The following reactions have the indicated equilibrium constants at a particular temperature:N2(g)+ O2(g)⇌ 2NO(g)Kc = 8.3 ×10−252NO(g)+ O2(g)⇌ 2NO2(g)Kc = 6.4 ×109Determine the value of the equilibrium constant for the following equation at the same temperature:2NO(g)⇌ N2(g)+ O2(g)

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:00, mapoohdoll
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 13:00, yaneiryx5476
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

Chemistry, 22.06.2019 14:30, clemsongirl5392
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2
You know the right answer?
Enter your answer in the provided box. The following reactions have the indicated equilibrium consta...
Questions in other subjects:


Mathematics, 05.10.2020 15:01



Chemistry, 05.10.2020 15:01

Mathematics, 05.10.2020 15:01

Mathematics, 05.10.2020 15:01


Social Studies, 05.10.2020 15:01

Mathematics, 05.10.2020 15:01

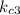 =1.21*
=1.21*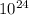
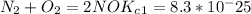 ...................1
...................1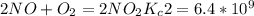 ………..2
………..2
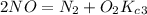 =? ………………………3
=? ………………………3