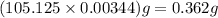Chemistry, 10.03.2020 06:47 payshencec21
A student titrated a weak acid with 0.0994 M NaOH solution. It took the student 34.65mL of NaOH to reach the equivalence point. How many grams of weak acid did the student use for titration, if molar mass of the weak acid was 105.125 g/mol

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:30, microwave13016
Agroup of students is studying convection current. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other is in an area with warm air. after 10 minutes, the balloon are released from a height of 1 meter. which of the following to the students most likely observe? a) the warm balloon expands and rises. the cold balloon shrinks and sinks b) the balloon both rise. the cold balloon is larger than the warm balloon c) the cold balloon expands and rises. the warm balloon shrinks and sinks d) the balloon rise at the same rate. both balloons are the same size
Answers: 1

Chemistry, 22.06.2019 09:00, heids17043
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3

Chemistry, 22.06.2019 16:10, 00015746
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2
You know the right answer?
A student titrated a weak acid with 0.0994 M NaOH solution. It took the student 34.65mL of NaOH to r...
Questions in other subjects:







Geography, 22.10.2020 01:01

Arts, 22.10.2020 01:01