Chemistry, 10.03.2020 06:21 joseroblesrivera123
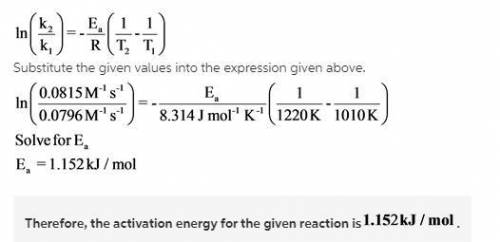
Understanding the high-temperature behavior of nitrogen oxides is essential for controlling pollution generated in automobile engines. The decomposition of nitric oxide (NO)(NO) to N2N2 and O2O2 is second order with a rate constant of 0.0796 M−1⋅s−1M−1⋅s−1 at 737∘C∘C and 0.0815 M−1⋅s−1M−1⋅s−1 at 947∘C∘C.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 23.06.2019 09:40, ventalexander8406
Write balanced nuclear equations for the formation of five elements whose atomic number is between helium (2) and iron (26):
Answers: 1

Chemistry, 23.06.2019 13:20, TayJoker966
What volume of 24% trichloroacetic acid (tca) is needed to prepare eight 3 ounce bottles of 10% tca solution?
Answers: 2

Chemistry, 23.06.2019 15:00, swelch2010
The atoms in a have a definite volume, but move quickly enough to overcome the forces of attraction between them. a. solid b. liquid c. gas
Answers: 2
You know the right answer?
Understanding the high-temperature behavior of nitrogen oxides is essential for controlling pollutio...
Questions in other subjects:

English, 03.03.2021 19:20

Mathematics, 03.03.2021 19:20

Health, 03.03.2021 19:20

Biology, 03.03.2021 19:20



Mathematics, 03.03.2021 19:20


Mathematics, 03.03.2021 19:20

Arts, 03.03.2021 19:20