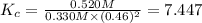Chemistry, 10.03.2020 05:58 emilie3849
A mixture initially contains A, B, and C in the following concentrations: [A] = 0.500 M , [B] = 0.800 M , and [C] = 0.350 M . The following reaction occurs and equilibrium is established: A+2B⇌C At equilibrium, [A] = 0.330 M and [C] = 0.520 M . Calculate the value of the equilibrium constant, Kc.

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 08:00, juliannxkim
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 09:20, kevinhernandez582
What will most likely happen when two bromine atoms bond together?
Answers: 3
You know the right answer?
A mixture initially contains A, B, and C in the following concentrations: [A] = 0.500 M , [B] = 0.80...
Questions in other subjects:

History, 30.07.2019 14:00

History, 30.07.2019 14:00






Mathematics, 30.07.2019 14:00


![K_c=\frac{[C]}{[A][B]^2}](/tpl/images/0540/1386/240ef.png)