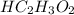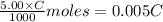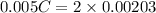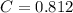Chemistry, 10.03.2020 05:58 ayoismeisalex
A 5.00 mL sample of vinegar, an aqueous solution of acetic acid (HC2H3O2), is titrated with 0.0599 M Ca(OH)2, and 33.93 mL of the Ca(OH)2 solution is required to reach the equivalence point. What is the molarity of the acetic acid? Group of answer choices

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 19:40, powberier6979
What is the wavelength of a 3*10^12 hz infrared wave a 3*10^20m b 1* 10^4m c 3*10^-3m d 1*10^-4 m
Answers: 1

Chemistry, 23.06.2019 02:00, xoxoadara13ox07ck
Now look at the segment of the graph between the two data points marked with black squares. describe how the boiling point and melting point plots behave between these points. be as specific as possible.
Answers: 1
You know the right answer?
A 5.00 mL sample of vinegar, an aqueous solution of acetic acid (HC2H3O2), is titrated with 0.0599 M...
Questions in other subjects:





Mathematics, 27.07.2020 02:01

Mathematics, 27.07.2020 02:01



Mathematics, 27.07.2020 02:01


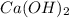 neutralizes 2 moles of
neutralizes 2 moles of