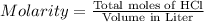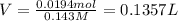Calculate the maximum volume (in mL) of 0.143 M HCl that each of the following antacid formulations would be expected to neutralize. Assume complete neutralization.
a. A tablet containting 350 mg Al(OH)3 and 250 mg Mg(OH)2.
b. A tablet containing 970 mg of CaCO3.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:10, vapelordcarl69
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3


Chemistry, 22.06.2019 13:30, suemmimonjaras8374
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1
You know the right answer?
Calculate the maximum volume (in mL) of 0.143 M HCl that each of the following antacid formulations...
Questions in other subjects:

Mathematics, 08.12.2020 02:30

English, 08.12.2020 02:30


Mathematics, 08.12.2020 02:30

Mathematics, 08.12.2020 02:30

Mathematics, 08.12.2020 02:30




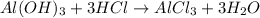
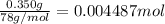
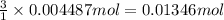 of HCl.
of HCl.
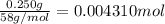
 of HCl.
of HCl.
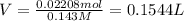
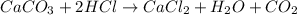
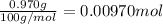
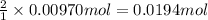 of HCl.
of HCl.