
Chemistry, 10.03.2020 04:41 flynwildozfuf5
Consider the reaction: N2(g) + 3H2(g) → 2NH3(g) Suppose that a particular moment during the reaction, molecular hydrogen is reacting at a rate of −0.0160 M/s. At what rate is ammonia being formed?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, megaaan214p61pb7
Which compounds have the empirical formula ch2o? a. c2h4o2 b. c3h6o3 c. ch2o2 d. c5h10o5 e. c6h12o6
Answers: 3

Chemistry, 22.06.2019 05:00, adrian128383
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 07:30, SchoolFirst9811
The scheme below is from a series of reactions that are part of a synthesis of vitamin a. answer the following questions with reference to this scheme. (i) what is "reagent a"? (ii) draw a step-by-step mechanism which explains the formation of compound c from compound b (iii) which reagents would you use to form compound e from compounds c and d (reagents b and c)? for each reagent suggested above in (ii) explain the role of the reagent in the reaction to (iv) form compound e. you may wish to do this by drawing a mechanism. 1. addition of reagent a но reagent a 2. н, о" thо oh нон-с compound a. compound b. compound c .ch-оh 1. reagent b "сно 2. reagent c сh oh compound e. compound d.
Answers: 2

Chemistry, 22.06.2019 09:30, strevino9178
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1
You know the right answer?
Consider the reaction: N2(g) + 3H2(g) → 2NH3(g) Suppose that a particular moment during the reaction...
Questions in other subjects:

Mathematics, 03.02.2020 10:44

History, 03.02.2020 10:44

Mathematics, 03.02.2020 10:44




Mathematics, 03.02.2020 10:44

History, 03.02.2020 10:44

Social Studies, 03.02.2020 10:44

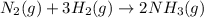
![R=\frac{-1}{1}\frac{d[N_2]}{dt}=\frac{-1}{3}\frac{d[H_2]}{dt}=\frac{1}{2}\frac{d[NH_3]}{dt}](/tpl/images/0539/9987/a62ba.png)
![-\frac{d[H_2]}{dt}=0.0160M/s](/tpl/images/0539/9987/d5dae.png)
![-\frac{d[NH_3]}{dt}](/tpl/images/0539/9987/527e4.png)
![\frac{-1}{3}\frac{d[H_2]}{dt}=\frac{1}{2}\frac{d[NH_3]}{dt}](/tpl/images/0539/9987/2a2d1.png)
![\frac{-1}{3}\frac{d[H_2]}{dt}\times 2=\frac{d[NH_3]}{dt}](/tpl/images/0539/9987/92027.png)
![\frac{1}{3}(-\frac{d[H_2]}{dt})\times 2=\frac{d[NH_3]}{dt}](/tpl/images/0539/9987/e9e1c.png)



