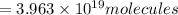Hydrogen gas, H2, reacts with nitrogen gas, N2, to form ammonia gas, NH3, according to the equation
3H2(g)+N2(g)→2NH3(g)
NOTE: Throughout this tutorial use molar masses expressed to five significant figures.
Part A
How many moles of NH3 can be produced from 18.0 mol of H2 and excess N2?
Part B
How many grams of NH3 can be produced from 4.50 mol of N2 and excess H2.
Part C
How many grams of H2 are needed to produce 12.57 g of NH3?
Part D
How many molecules (not moles) of NH3 are produced from 1.99×10−4 g of H2?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:10, hannacarroll2539
The enthalpy of formation of water is -285.8 kj/mol. what can be inferred from this statement?
Answers: 1

Chemistry, 22.06.2019 20:00, montimcdaniel
If one fission reaction of a uranium-235 atom produced two neutrons, how many neutrons would be released if the chain reaction occurred three more times?
Answers: 1

Chemistry, 23.06.2019 10:30, jetblackcap
An atom that gains or loses one or more electrons is called a(n)
Answers: 1
You know the right answer?
Hydrogen gas, H2, reacts with nitrogen gas, N2, to form ammonia gas, NH3, according to the equation<...
Questions in other subjects:


Mathematics, 15.03.2020 23:51

Mathematics, 15.03.2020 23:51


Mathematics, 15.03.2020 23:52

Mathematics, 15.03.2020 23:53

Mathematics, 15.03.2020 23:53

English, 15.03.2020 23:53

Mathematics, 15.03.2020 23:53

SAT, 15.03.2020 23:54

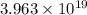 molecules of ammonia will be produced.
molecules of ammonia will be produced.
 of ammonia
of ammonia of ammonia
of ammonia
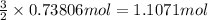 of hydrogen gas
of hydrogen gas 
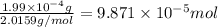
 moles of hydrogen gas will give ;
moles of hydrogen gas will give ; of ammonia.
of ammonia. molecules/ atoms
molecules/ atoms .
.