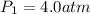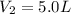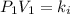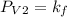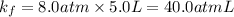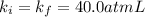Chemistry, 10.03.2020 04:53 gabbym39077
A certain gas is present in a 10.0 LL cylinder at 4.0 atmatm pressure. If the pressure is increased to 8.0 atmatm the volume of the gas decreases to 5.0 LL . Find the two constants kiki, the initial value of kk, and kfkf, the final value of kk, to verify whether the gas obeys Boyle’s law by entering the numerical values for kiki and kfkf in the space provided.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 22:30, kiki197701
Agas at 155 kpa and standard temperature has an initial volume of 1.00 l. the pressure of the gas rises to 500 kpa as the temperature also rises to 135°c. what is the new volume? 2.16 l 0.463 l 0.207 l 4.82 l
Answers: 3


Chemistry, 22.06.2019 13:00, monkeyrose1999
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3
You know the right answer?
A certain gas is present in a 10.0 LL cylinder at 4.0 atmatm pressure. If the pressure is increased...
Questions in other subjects:

Chemistry, 15.02.2022 21:00






Mathematics, 15.02.2022 21:00


Chemistry, 15.02.2022 21:00

Mathematics, 15.02.2022 21:00

 both are equal to 40.0 atm L.
both are equal to 40.0 atm L.