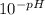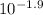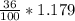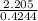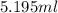Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:20, Jessicadiaz8602
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1


Chemistry, 22.06.2019 16:50, shaylawaldo11
Which element is least likely to undergo a chemical reaction
Answers: 3

Chemistry, 22.06.2019 22:30, arodavoarodavo
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3
You know the right answer?
What volume of a concentrated HCl solution, which is 36.0% HCl by mass and has a density of 1.179 g/...
Questions in other subjects:

Physics, 21.06.2021 18:50

Mathematics, 21.06.2021 18:50

Computers and Technology, 21.06.2021 18:50


Mathematics, 21.06.2021 18:50



Mathematics, 21.06.2021 18:50

Mathematics, 21.06.2021 18:50