
Chemistry, 10.03.2020 04:52 Katie123amazing
Calculate ΔH∘f for NO(g) at 435 K, assuming that the heat capacities of reactants and products are constant over the temperature interval at their values at 298.15 K. Molar heat capacities of NO(g), N2(g), and O2(g) at 298.15 K are 29.86, 29.13, and 29.38 J⋅K−1⋅mol−1. The standard enthalpy of formation of NO(g) is 91.3 kJ⋅mol−1 at 298.15 K.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:20, kkakk19
Calculate the enthalpy of the following reaction: 4 b (s) + 3 o2 (g) → 2 b2o3 (s) given the following pertinent information: (a) b2o3 (s) + 3 h2o (g) → 3 o2 (g) + b2h6 (g), δhoa = +2035 kj (b) 2 b (s) + 3 h2 (g) → b2h6 (g), δhob = +36 kj (c) h2 (g) + latex: \frac{1}{2} 1 2 o2 (g) → h2o (l), δhoc = −285 kj (d) h2o (l) → h2o (g), δhod = +44 kj
Answers: 3


Chemistry, 23.06.2019 01:00, williedenmark42
What is the most common form of matter in the universe
Answers: 2

Chemistry, 23.06.2019 10:50, broyochey1
Which compound should undergo substitution of the bromine by phenolate anion? draw the structure of the organic product?
Answers: 1
You know the right answer?
Calculate ΔH∘f for NO(g) at 435 K, assuming that the heat capacities of reactants and products are c...
Questions in other subjects:

Mathematics, 06.04.2021 23:10

Mathematics, 06.04.2021 23:10



Chemistry, 06.04.2021 23:10

Mathematics, 06.04.2021 23:10

Mathematics, 06.04.2021 23:10


Mathematics, 06.04.2021 23:20

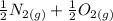 ------>
------>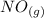
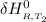 =
= 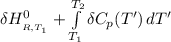
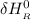 = enthalpy of reaction
= enthalpy of reaction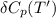 = the difference in the heat capacities of the products and the reactants.
= the difference in the heat capacities of the products and the reactants.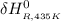 =
= 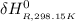

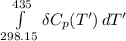
![1(91300 J.mol^{-1} ) +\int\limits^{435}_{298.15} [{(29.86)-\frac{1}{2}(29.38)-\frac{1}{2}29.13}]J.K^{-1}.mol^{-1} \, dT'](/tpl/images/0540/0534/3b971.png)



