
Chemistry, 10.03.2020 04:01 claudiasfandom
A piece of dry ice (solid CO2) weighing 17.0 g is placed in a 0.500−L bottle filled with air at 0.947 atm and 492.7°C. The bottle is capped, and the dry ice changes to gas. What is the final pressure inside the bottle

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:00, sabahfayaskhan
Which of the following statements is true? question 4 options: nuclear decay rates vary with the conditions of the reaction, but chemical reaction rates do not. chemical reaction rates vary with the conditions of the reaction, but nuclear decay rates do not. neither chemical reaction rates nor nuclear decay rates vary with the conditions of the reaction. both chemical reaction rates and nuclear decay rates vary with the conditions of the reaction.
Answers: 1


Chemistry, 22.06.2019 02:40, hardwick744
Achange in the number of neutrons in an atom will change an blank . when the number of protons changes in an atom, a new element will form.
Answers: 2

You know the right answer?
A piece of dry ice (solid CO2) weighing 17.0 g is placed in a 0.500−L bottle filled with air at 0.94...
Questions in other subjects:


Chemistry, 03.12.2020 20:40



Mathematics, 03.12.2020 20:40

Social Studies, 03.12.2020 20:40

Mathematics, 03.12.2020 20:40


History, 03.12.2020 20:40

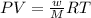
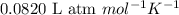
![492.7^oC=[492.7+273]K=765.7K](/tpl/images/0539/8543/60167.png)



