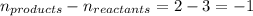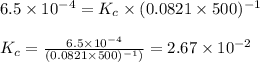Chemistry, 10.03.2020 03:32 haydoc1025
The equilibrium constant, Kp, has a value of 6.5 × 104 at 308 K for the reaction of nitrogen monoxide with chlorine. 2NO(g) + Cl2(g) 2NOCl(g) [Balanced] What is the value of Kc

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, joelpimentel
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2

Chemistry, 22.06.2019 08:30, Apple557
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1

Chemistry, 22.06.2019 13:00, torigirl4126
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3

Chemistry, 22.06.2019 19:30, Adrian12313
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1
You know the right answer?
The equilibrium constant, Kp, has a value of 6.5 × 104 at 308 K for the reaction of nitrogen monoxi...
Questions in other subjects:


Medicine, 28.03.2021 02:20







Mathematics, 28.03.2021 02:20

Mathematics, 28.03.2021 02:20

 for the given reaction is
for the given reaction is 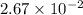
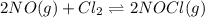
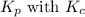 is given by the formula:
is given by the formula: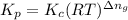
 = equilibrium constant in terms of partial pressure =
= equilibrium constant in terms of partial pressure = 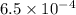
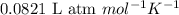
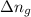 = change in number of moles of gas particles
= change in number of moles of gas particles