
Part A Which of the following statements about chemical formulas is FALSE? Which of the following statements about chemical formulas is FALSE? The subscripts represent the relative mass of each type of atom in the compound. Different compounds made of the same elements have different subscripts. The subscripts represent the relative number of each type of atom in the compound. The subscripts do not change for a given compound. All of the statements are true.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, isabelvaldez123
The boiling point of liquids is very high what does it indicate
Answers: 1

Chemistry, 22.06.2019 05:20, anggar20
Why does the sun appear to be the brightest star in the sky? a- its apparent brightness is much greater than other stars. b- it burns more gas, making it brighter than any other star. c- it is the largest star in the galaxy, so it is the brightest star. d- its relative distance to earth is closer than the other stars.
Answers: 1


Chemistry, 22.06.2019 12:30, Svetakotok
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2
You know the right answer?
Part A Which of the following statements about chemical formulas is FALSE? Which of the following st...
Questions in other subjects:










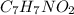 the subscripts present in it are 7, 7, 1, and 2.
the subscripts present in it are 7, 7, 1, and 2.


