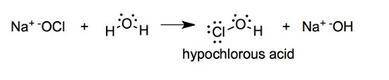
Once the ionic solid has dissolved, the anion that is formed is able to react as a base, with water as the acid. Write the net acid-base reaction that occurs when dissolved NaClO reacts with water. (Use the lowest possible coefficients. Omit states-of-matter in your answer.)

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, deaishaajennings123
What is the equilibrium constant of aa+bb=cc+dd
Answers: 1


Chemistry, 22.06.2019 16:00, graciewyatt6833
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2

Chemistry, 22.06.2019 18:10, sangamlama
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1
You know the right answer?
Once the ionic solid has dissolved, the anion that is formed is able to react as a base, with water...
Questions in other subjects:


Mathematics, 03.02.2022 03:50


Mathematics, 03.02.2022 03:50



Mathematics, 03.02.2022 03:50

Mathematics, 03.02.2022 03:50


French, 03.02.2022 04:00